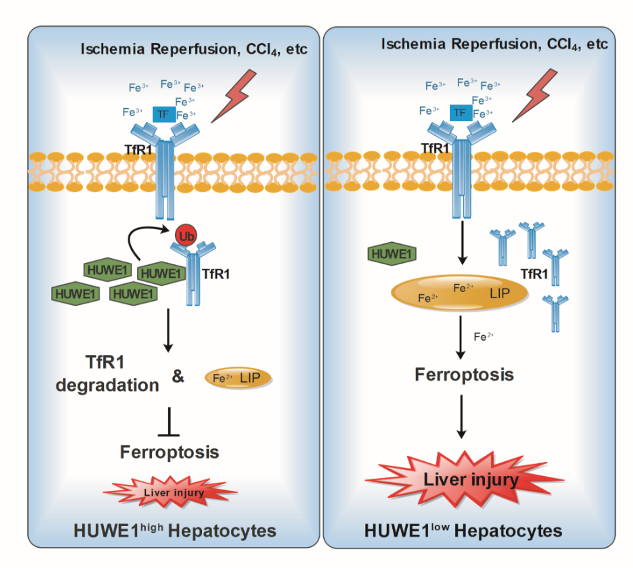
The article Ubiquitin ligase E3 HUWE1/MULE targets transferrin receptor for degradation and suppresses ferroptosis in acute liver injury was published in Cell Death & Differentiation by PI Jing Zhang/Professor Qing Zhong/Professor Qiang Xia Lab of Basic Medical Sciences and Renji Hospital on 8 March 2022, analyzed ubiquitination and regulation of transferrin receptor TfR1, a key ferroptosis protein in acute liver injury, and provided a new idea for alleviating acute liver injury during liver transplantation.
Abstract
Hepatic ischemia followed by reperfusion (I/R), a major clinical problem during liver surgical procedures, can induce liver injury with severe cell death including ferroptosis which is characterized by iron-dependent accumulation of lipid peroxidation. The HECT domain-containing ubiquitin E3 ligase HUWE1 (also known as MULE) was initially shown to promote apoptosis. However, our preliminary study demonstrates that high expression of HUWE1 in the liver donors corelates with less injury and better hepatic function after liver transplantation in patients. Thus, we investigate the role of HUWE1 in acute liver injury, and identify HUWE1 as a negative ferroptosis modulator through transferrin receptor 1(TfR1). Deficiency of Huwe1 in mice hepatocytes (HKO) exacerbated I/R and CCl4-induced liver injury with more ferroptosis occurrence. Moreover, Suppression of Huwe1 remarkably enhances cellular sensitivity to ferroptosis in primary hepatocytes and mouse embryonic fibroblasts. Mechanistically, HUWE1 specifically targets TfR1 for ubiquitination and proteasomal degradation, thereby regulates iron metabolism. Importantly, chemical and genetic inhibition of TfR1 dramatically diminishes the ferroptotic cell death in Huwe1 KO cells and Huwe1 HKO mice. Therefore, HUWE1 is a potential protective factor to antagonize both aberrant iron accumulation and ferroptosis thereby mitigating acute liver injury. These findings may provide clinical implications for patients with the high-expression Huwe1 alleles.
Link: https://www.nature.com/articles/s41418-022-00957-6
About Lab
Laboratory of Autophagy and Necrosis in Human Diseases
Qing Zhong’s lab has long been devoted to the study of biochemical regulation of autophagy in mammalian cells. Autophagy is a process by which cells process and recycle their components through lysosomes. Autophagy is essential for maintenance of cellular homeostasis and its dysfunction has been linked to major human diseases, including cancer, neurodegeneration, and immune diseases. Zhong’s group is specifically interested in dissecting the biochemical mechanisms of autophagy, including lipid modification, lipid transfer, autophagic membrane elongation, tethering, fusion, and membrane integrity sensing and repair. Zhong’s group has made many achievements in this field. The group has elaborated on formation of autophagosomes (PNAS 2008, 2010, 2011), recognition of autophagic substrates (Nature 2013), and fusion of autophagosomes with lysosomes (Mol Cell 2012, Nature 2015, JCB 2022). Understanding these key regulatory steps in autophagy will allow us to develop new therapeutic tools for human diseases. Furthermore, Zhong’s group is also interested in dissecting the biochemical mechanism and physiological functions of emerging necrotic cell death pathways, and their interconnection with autophagy.
Laboratory of Oxidative Stress Induced Necrosis and Human Diseases
Programmed cell death is crucial for multiple physiological processes of multicellular organisms, and its dysregulation is closely related to a variety of human diseases, such as cancers, neurodegenerative diseases and ischemic diseases. Recent evidence shows that programmed cell death is not only limited to apoptosis, but also necrosic cell death are comprehensively regulated. Therefore, the role and molecular mechanisms of programmed cell death in human diseases and its implications in clinical therapy have always been the focus of our research.Dr. Jing Zhang's group utilizes biochemistry, molecular and cell biology, model animals, and compound screening to dissect the molecular mechanisms of oxidative stress induced necrotic cell death, and explore its clinical potentials for ischemic diseases and fibrotic diseases. It mainly contains the following aspects:
1) Post-translational modification in ferroptosis and its effect in liver diseases (Cell Death Differ, 2022);
2) The role of ferroptosis in cellular metabolism, cancer metastasis, and cancer therapy.
Qiang Xia Lab
The research programs are currently on liver malignancy quick growth and metastasis, infection and immune regulation in acute-on-chronic liver failure (ACLF), ischemia-reperfusion injury (IRI), liver regeneration, immune monitoring, organ preservation and repair, etc. In recent years, we successfully obtained support for 1 program under the National Key Research and Development Program of China, 1 program under Major research program of National Natural Science Foundation of China, 38 other programs under the National Natural Science Fund Project, led 3 multiple center clinical trials in China, and gained the total research funding of more than RMB 73 million. We published 200 clinical and scientific research papers in journals such as Chemical Reviews, Nat Rev Immunol, Gastroenterology, Hepatology, Liver Transplantation, etc. The research achievements in the discipline have also been awarded Second Prize of National Science and Technology Progress, the first prize of Science and Technology Progress of National Ministry of Education, the First Prize of Shanghai Scientific and Technology, the First Prize of Chinese Medicine Scientific and Technology and the First Prize of Shanghai Medical Science and Technology Progress.