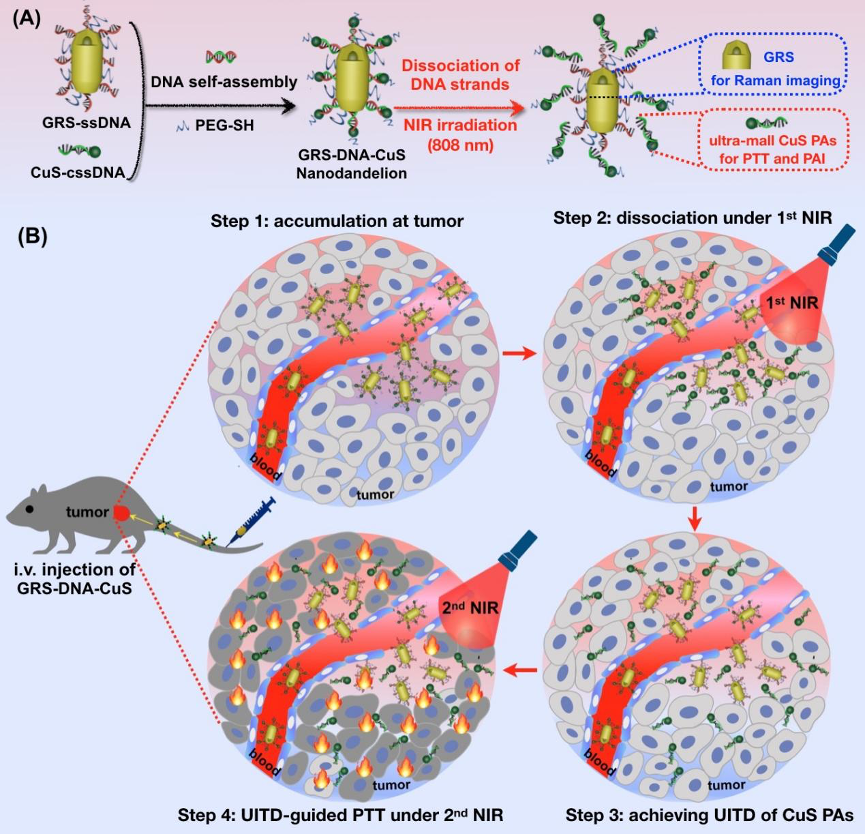
The article DNA-assembled visible nanodandelions with explosive hydrogen-bond breakage achieving uniform intra-tumor distribution (UITD)-guided photothermal therapy was published in Biomaterials by Professor Zeyu Xiao Lab of Basic Medical Sciences in March 2022, designed a visualized nanomachine similar to dandelion structure based on DNA self-assembly technology and named it DNA-assembled visible nanodandelions. The nanodandelions used the explosive pyrolytic dissociation property of hydrogen bond in DNA double strand, dissociating the photothermal reagent from large particle size to small particle size in tumor, visually monitoring its spatio-temporal uniform dispersion process in tumor tissue, and significantly improving the photothermal therapy efficacy.
Abstract
Photothermal therapy (PTT) has received increasing attention for treating tumors. However, a long-standing challenge in PTT is non-uniform distribution of photothermal agents (PAs) in tumor tissues, resulting in limited therapeutic efficiency. Herein, inspired by dandelions blowing away by the wind, we have designed a DNA-assembled visible GRS-DNA-CuS nanodandelion, which can achieve uniform intra-tumor distribution (UITD) of PAs, thus enhancing the photothermal therapeutic efficiency. GRS-DNA-CuS is featured by the formation of hydrogen bond between the core of single-strand DNA-modified Raman nanoprobes (GRS) and the shell of complementary single-strand DNA-modified CuS PAs. Under Raman imaging-guided 1st NIR irradiation, hydrogen bond in GRS-DNA-CuS is explosively broken, resulting in large-sized GRS-DNA-CuS (∼135 nm) be completely dissociated into GRS and ultra-small CuS PAs (∼12 nm) within 1 min. Such an explosive dissociation instantly enhances the local concentration of ultra-small CuS PAs and slightly rises intra-tumor temperature, thus increasing the diffusion coefficient of PAs and promoting their UITD. This UITD of CuS PAs enhances the photothermal anti-tumor effects. Three out of five tumors are completely eliminated under photoacoustic imaging-guided 2nd NIR irradiation. Overall, this study provides one UITD-guided PTT strategy for highly effective tumor treatment by exerting explosive breakage property of hydrogen bond, broadening the application scope of DNA-assembly technique in oncology field.
Link: https://www.sciencedirect.com/science/article/pii/S0142961222000205
About Lab
Laboratory of Visualized Drug Delivery
The group has been dedicated to develop visualized drug delivery systems with high sensitivity and high selectivity for disease diagnosis and therapy, by using interdisciplinary technologies and methods (e.g. chemistry, biology, pharmacology and molecular imaging). The specific research contents are as follows:
(1) Developing visualized drug delivery systems based on in-vivo optical imaging (such as Raman imaging) or nuclear medical imaging technologies, for intra-operative diagnosis and removal of residual micro-tumors, reversing targeted-drug resistance, medication guidance and treatment evaluation;
(2) Investigating oral drug delivery system for naked-eye visualization and treatment of colon diseases at the early stage;
(3) Designing DNA-modularied programmable bivalent drugs for precise receptor regulation.
Recently, we have published more than 40 research articles in the international academic journals (e.g. Chem Soc. Rev., Nature Comm. Angew. Chem., Nano Lett, ACS Nano, Adv Funct Mater., etc), and three Book Chapters. Among them, there has been 1 article cited more than 1000 times, 5 articles cited more than 200 times, 7 articles cited more than 100 times, and 3 articles selected as the highly cited papers by ESI. One visualized drug delivery system has begun clinical trial stage. In addition, we also have applied/authorized two international patents and one domestic patent.