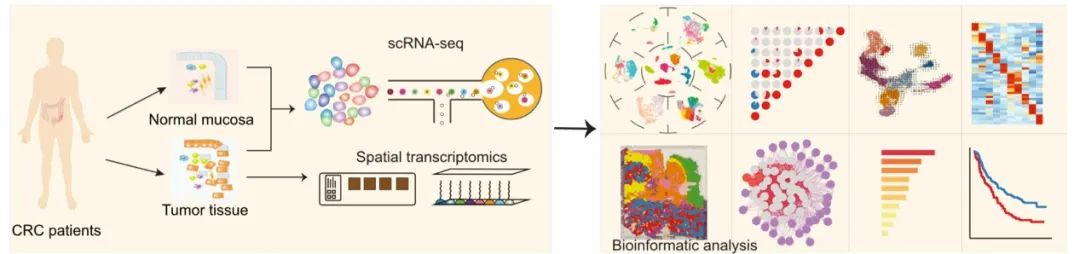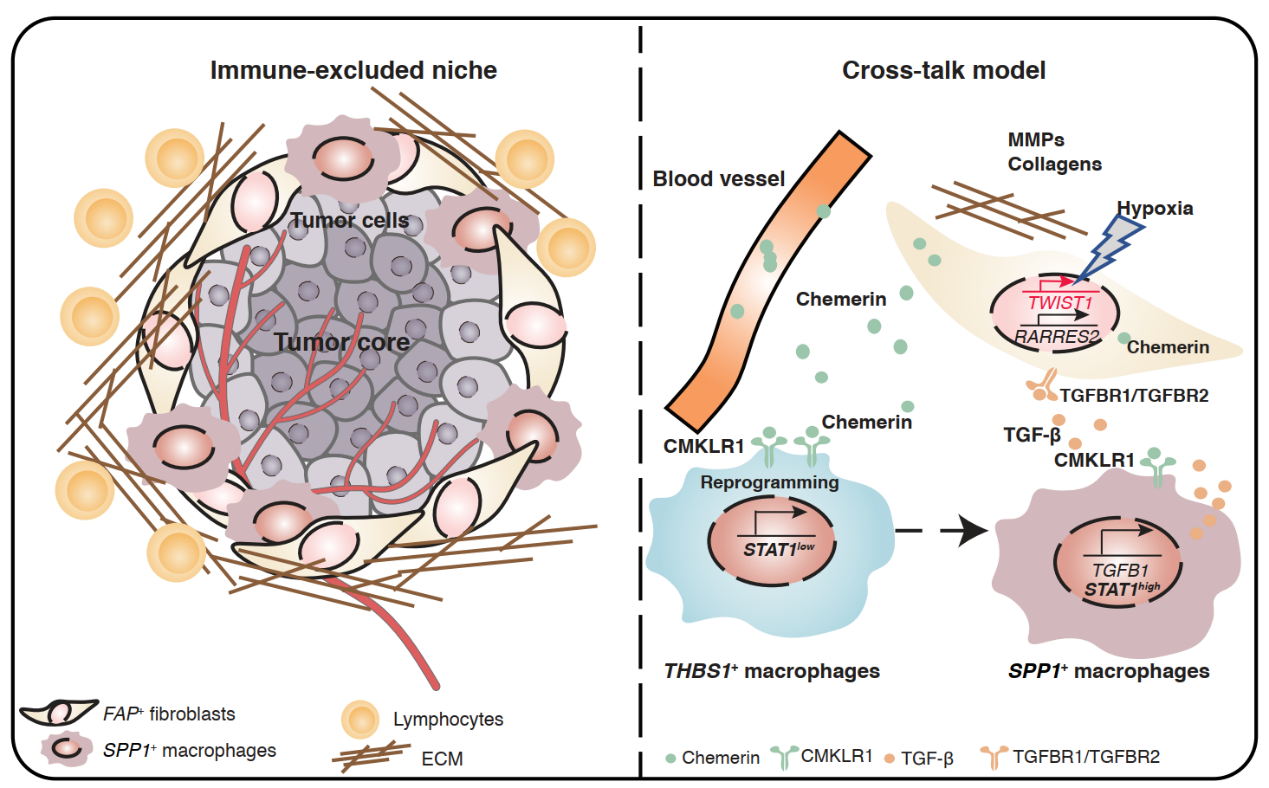
The article Single-cell and spatial analysis reveal interaction of FAP+ fibroblasts and SPP1+ macrophages in colorectal cancer was published in Nature Communications by Professor Bing Su Lab and PI Youqiong Ye Lab of /Shanghai Institute of Immunology on 1 April 2022, found that FAP+ fibroblasts and SPP1+macrophages were infiltrated significantly in CRC and correlated with poor prognosis and tumor immunotherapy tolerance based on the analysis of single-cell multi-omics, spatial transcriptome sequencing and immunofluorescence imaging.
Abstract
Colorectal cancer (CRC) is among the most common malignancies with limited treatments other than surgery. The tumor microenvironment (TME) profiling enables the discovery of potential therapeutic targets. Here, we profile 54,103 cells from tumor and adjacent tissues to characterize cellular composition and elucidate the potential origin and regulation of tumor-enriched cell types in CRC. We demonstrate that the tumor-specific FAP+ fibroblasts and SPP1+ macrophages were positively correlated in 14 independent CRC cohorts containing 2550 samples and validate their close localization by immuno-fluorescent staining and spatial transcriptomics. This interaction might be regulated by chemerin, TGF-β, and interleukin-1, which would stimulate the formation of immune-excluded desmoplasic structure and limit the T cell infiltration. Furthermore, we find patients with high FAP or SPP1 expression achieved less therapeutic benefit from an anti-PD-L1 therapy cohort. Our results provide a potential therapeutic strategy by disrupting FAP+ fibroblasts and SPP1+ macrophages interaction to improve immunotherapy.
Link: https://www.nature.com/articles/s41467-022-29366-6
About Lab
Laboratory of Mucosal Immune Regulation
The research in the laboratory focuses on the biology of signal transduction mediated by the intracellular protein kinase network such as the mitogen-activated protein kinase (MAPK) cascades and the mammalian target of rapamycine (mTOR) pathway in gut immune responses and blood vessel function/development. The current studies have focused on the regulatory mechanism of MAPK and mTOR signaling pathways in intestinal stromal cells, macrophages, T cells and innate lymphoid cells and their roles in intestinal and lung inflammation, cancer, and intestinal fibrosis. The recent research work of the laboratory has been published in journals such as Nature (2021), Cell (2021,2019), Nature Communications (2022,2018), EMBO J (2020), Molecular Cell (2017).
Laboratory of Tumor Multiomics and Immunotherapy
The research in the laboratory focuses on tumor microenvironment (TME) and immunotherapy. They take full advantage of the multi-omics data in human cancer, combine with innovative bioinformatics methods, basic experiments, clinical cohorts and other multi-scale methods to build an immunotherapy efficacy prediction model, to explore the regulatory roles of tumor (boundary) microenvironment on tumor progression and immune escape, providing new potential therapeutic strategies for immunotherapy (Cancer Cell, 2020; Nature Metabolism, 2019; Nature Immunology, 2019; Nature Communications, 2020, 2022; etc.). The main research contents as follows:
1) Combining multi-scale data such as spatial transcriptomics, single-cell multi-omics, pathological images, etc., to construct approaches to accurately study the regulation roles of tumor boundary microenvironment in tumor immune microenvironment subtypes, metastasis, and immunotherapy resistance, and to develop new therapeutic strategies based on the targets in tumor boundary microevironment;
2) Single-cell and other omics based clinical cohorts reveal tumor microenvironment-related characteristics to assist tumor immunotherapy.