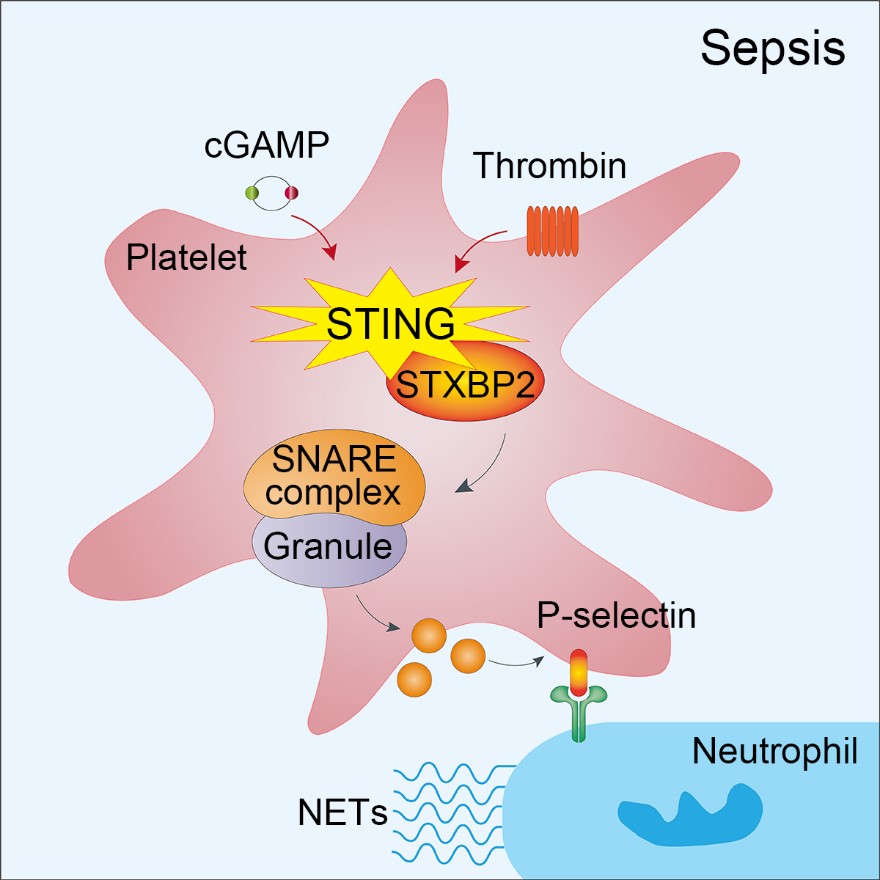
The article STING activation in platelets aggravates septic thrombosis by enhancing platelet activation and granule secretion was published in Immunity by Professor Junling Liu / Yanyan Xu Lab of Shanghai Jiao Tong University School of Medicine on March 20, 2023, systematically revealed the deep mechanism of how platelets respond to bacterial and other microbial infection stimulation and the release of platelet particles. In this study, the researchers found that platelet STING loss can alleviate the formation of microthrombe and NETs structures in the liver and lungs of mice induced by sepsis with vivo imaging technology, decrease the indicators related to liver and lung injury and abnormal coagulation, and enhance the survival rate of mice. In vitro experiments, STING agonist cGAMP was found to enhance the platelets' response to irritant agent like thrombin and collagen. However, as a class of non-nucleated cells, platelets is lack of transcription process. The authors found that cGAMP could not turn on STING/TBK1/IRF3 signal in platelets, but could promote the palmitoylation of STING, suggesting that there was a new downstream activation signal of STING in platelets that was different from STING/TBK1/IRF3.

Abstract:
Sepsis is a dysregulated inflammatory consequence of systemic infection. As a result, excessive platelet activation leads to thrombosis and coagulopathy, but we currently lack sufficient understanding of these processes. Here, using the cecal ligation and puncture (CLP) model of sepsis, we observed septic thrombosis and neutrophil extracellular trap formation (NETosis) within the mouse vasculature by intravital microscopy. STING activation in platelets was a critical driver of sepsis-induced pathology. Platelet-specific STING deficiency suppressed platelet activation and granule secretion, which alleviated sepsis-induced intravascular thrombosis and NETosis in mice. Mechanistically, sepsis-derived cGAMP promoted the binding of STING to STXBP2, the assembly of SNARE complex, granule secretion, and subsequent septic thrombosis, which probably depended on the palmitoylation of STING. We generated a peptide, C-ST5, to block STING binding to STXBP2. Septic mice treated with C-ST5 showed reduced thrombosis. Overall, platelet activation via STING reveals a potential strategy for limiting life-threatening sepsis-mediated coagulopathy.
About Lab:
Laboratory of Thrombus and Vascular Physiology
Adhesion molecules and their intracellular signals play an important role in physiology and pathophysiology. Our research group mainly focuses on the mechanism of major adhesion molecules on the cell surface, such as integrin and multiple EGF like domains family proteins, in the pathogenesis and development of cardiovascular diseases and tumors, and finds corresponding targeted therapies




