近日,国际胃肠病学权威期刊《Gastroenterology》在线发表了中科院上海生命科学研究院/欧洲杯竞猜平台健康科学研究所张笑人研究组的最新研究成果“A
microRNA 221- and 222-mediated feedback loop, via PDLIM2, maintains
constitutive activation of NFκB and STAT3 in colorectal cancer
cells”,揭示miR-221/22介导的炎症信号正反馈环路在结直肠癌发生和发展过程中的重要作用。
结直肠癌是胃肠道系统中最常见的恶性肿瘤之一,其发病率居恶性肿瘤第三位,患者死亡率高居恶性肿瘤死因第二位。近年来,我国结直肠癌的发病率和死亡率呈明显上升趋势。越来越多的证据表明,慢性炎症和结直肠癌的发生发展存在着密切的关系。其中,转录因子NFκB和STAT3两条通路的过度激活在慢性炎症和结直肠癌的发生发展过程中扮演着关键的作用。然而,目前导致这两条通路激活的分子机制仍不是十分清楚。
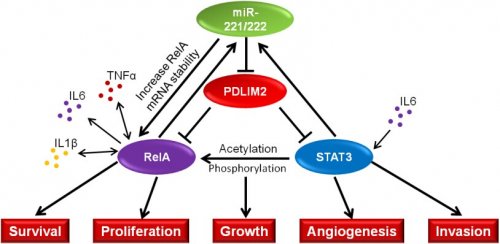
刘三宏副研究员和博士研究生孙小华在张笑人研究员的指导下与欧洲杯竞猜平台附属瑞金医院的王明亮教授合作研究发现,miR-221/222介导的正反馈环路促进NFκB和STAT3通路的激活,并促进结直肠癌的发生和发展。miR-221/222一方面通过稳定NFκB通路中的核心转录因子RelA的mRNA稳定性,维持RelA的高表达;另一方面通过抑制RelA和STAT3共有的E3泛素化连接酶PDLIM2,维持RelA和STAT3的蛋白稳定性,从而促进NFκB和STAT3通路的组成性激活。同时,NFκB和STAT3可以诱导miR-221/222的表达,由此形成正反馈环路,结果显示该反馈环路也存在于临床肿瘤样本中。在小鼠结肠癌AOM/DSS模型中,通过尾静脉注射能特异结合miR-221/222的“海绵”,可有效阻断NFκB和STAT3通路的激活,并抑制小鼠结肠癌的发生和发展。该研究发现了miR-221/222、
NFκB和STAT3正反馈环在结肠癌中的重要作用,为结肠癌的诊断和治疗提供了新的潜在靶点。
该研究得到了国家科技部、国家自然科学基金委和中国科学院等经费资助以及复旦大学附属中山医院侯英勇教授的大力支持和帮助。
A new mechanism underlying the constitutive activation of NFκB and
STAT3 in human colorectal cancer
Recently, the work from Dr. Xiaoren Zhang‘s group (Institute of
Health Sciences, Shanghai Institutes for Biological Sciences,
Chinese Academy of Sciences and Shanghai Jiao Tong University
School of Medicine), titled “A microRNA 221- and 222-mediated
feedback loop, via PDLIM2, maintains constitutive activation of
NFκB and STAT3 in colorectal cancer cells”, was published on line
in Gastroenterology. The study revealed that miR-221/222-mediated
positive feedback loop played an important role in the constitutive
activation of inflammatory signals and the development of
colorectal cancer.
Constitutive activation of NFκB and STAT3 pathways in human
colorectal cancers (CRCs) links inflammation to CRC development and
progression. However, the underlying mechanisms remain to be
elucidated. In collaboration with Prof. Mingliang Wang (Ruijin
Hospital Affiliated to Shanghai Jiao Tong University), Dr Sanhong
Liu and Ph. D candidate Xiaohua Sun, supervised by Dr. Xiaoren
Zhang, discovered that miR-221 and miR-222 positively regulated
both NFκB and STAT3 activities, which in return induced miR-221/222
expression, creating a positive feedback loop in human CRCs.
miR-221/222 directly bound to the coding region of RelA, leading to
increased RelA mRNA stability. In addition, miR-221/222 reduced
ubiquitination and degradation of RelA and STAT3 proteins by
directly targeting the 3’ UTR of PDLIM2 mRNA, an E3 ligase for both
RelA and STAT3, collectively upregulating RelA and STAT3 protein.
The study demonstrated that disruption of the positive feedback
loop suppressed human CRC cell growth in vitro and in vivo.
miR-221/222 expression correlated with the expression of RelA,
STAT3 and PDLIM2 in human CRCs. These findings defined a novel
mechanism underlying constitutive activation of NFκB and STAT3
pathways in human CRCs, revealing miR-221/222 served as novel
potential therapeutic targets for CRCs.
The project was supported by the Ministry of Science and
Technology of China, the National Natural Science Foundation of
China, China Academy of Sciences. Prof. Yingyong Hou (Zhongshan
Hospital Affiliated to Fudan University) also provided supports for
this project. (IHS)